Korea MFDS Certificate
Quasi-drugs Cosmetics

Purpose
The certification of 『Quasi-drugs/Cosmetics』is managed and supervised by the Ministry of Food and Drug Safety (MFDS). Its purpose is to comply with regulations regarding manufacturing, import, and sale, to prevent potential risks to public health through clinical trials or safety verification, to conduct on-site inspections for safety standards and human risk assessment, and to contribute to the improvement of public health and the development of the industry.
Overview
- Quasi-drugs
- As a system to prove compliance with the regulations required by the 「MFDS」, the goods designated by the Minister of Food and Drug Safety by Article 2 (7) of the Pharmaceutical Affairs Act and goods specified in the scope of quasi-drugs shall be notified and approved.
- Cosmetics
- As a system to prove conformity to the regulations required by the「MFDS」, the product shall be tested at a cosmetic testing institution to prove the safety of the product.
Target Products
Products subject to 「Quasi-drugs」
[ No. 1 ]
Products for personal hygiene sanitary
- Oral fresheners (internal use and mouthwash) / Deodorant · Antiperspirant (limited to external use) / Heat rash and erosion agents / Toothpastes / Feminine hygiene products
Mask
- For surgery / For health care / Droplet blocking
Sanitary products used for preservation, protection, and treatment of the affected (wound) area
- Eyepatches / Bandages / Elastic bandages / Plaster bandages / Cylindrical elastic bandages / Gauze / Cotton wools / Sticking plasters
[ No. 2 ]
Inhibitors such as bad breath
- Oral freshener (internal use and mouthwash) / Anti-underarm odor agents (limited to external use)/ Heat rash and erosion agents / Toothpastes
Repellents for mosquitoes and mites applied to the human body for human healthcare
Contact lens care products
Smoking cessation aid not containing nicotine
- Smoking craving depressants / Smoking habit improvement supplements
External disinfectant used directly on the human body
Ointment, Cataplasma, and spraypainrelief patch determined by standard manufacturing standards
Formulations for internal use
- Low-content vitamin and mineral preparations determined by the standard manufacturing standards, nourishing and tonic altering agents (oral liquids), gastric digestive agents (oral liquids), antidiarrheal (oral solids)
Preparations used for oral hygiene, etc.
- Topical liquids for cleaning and disinfecting dental root canals / Formulations to fix infants and children's thumb-sucking habits / Anti-snoring agents (adjuvant) / Teeth whitening agents / False teeth (dentures), orthodontic appliance cleaning/disinfectant / Plaque dye
[ No. 4 ]
Non-adhesive products used to absorb exudates from the affected area, such as pads, sponges, etc.
Sterilized items used for surgical treatment to prevent infection, such as sterile cotton swabs and sterile gloves
Mouth tissues for oral hygiene that cleans teeth and gums
Portable items used for inhalation by temporarily supplying air or oxygen before and after mountaineering, exercise, etc.
Items used for the sanitary treatment of bleeding and lochia (postpartum vaginal discharge) immediately after childbirth
Goods similar to those in each item of subparagraph
Products subject to「Cosmetics」
Products for infants and toddlers
- Shampoos and conditioners / Lotions and creams / Oils / Body cleaning products / Bath products
Products for bath
- Bath oils, tablets, capsules
Products for human body cleansing
- Foam cleansers / Body cleansers / Liquid soaps / Vulvar cleansers/ Water tissues (limited to human body cleaning)
Products for eye makeup
- Eyebrow Pencils / Eyeliners/ Eyeshadows / Mascaras
Products for aroma
- Perfumes / Powder fragrance / Sachets / Colognes/ Other products for aroma
Products for hair dyeing
- Hair tints / hair color sprays / Other products for hair dyeing
Products for color makeup
- Blushers / Face powders / Face cakes / Liquid cream cake foundations / Makeup bases / Makeup fixatives / Lipsticks / Lip liners / Lip glosses / Lip balms / Productsforbody painting / Productsforface painting / Makeup products / Other products for color makeup
Products for hair
- Hair conditioners / Scalptonics / Hair grooming aids / Hair creamsand• lotions / Hair oils / Hairsprays, Mousses, Waxes, Gels / Shampoos / Conditioners / Others products for hair
Products for nails
- Base coat / Undercoat / Nail polishes/ Nail enamels / Top coat/ Nail creams, Lotions, Essences / Removers for nail polishes and enamels / Other products for nails
Products for shaving
- Aftershave lotions / Talcum for men/ Pre-shave lotions / Shaving creams / Shaving foams / Other products for shaving
Products for basic makeup
- Astringent•soft•nourishing lotions / Massage cream essence oils / Powders / Body products / Packs / Masks / Eye products / Lotions / Creams / Skin softening products for hands and feet / Makeup removers such as cleansing water, oils, lotions, creams, etc. / Other products for basic makeup
Products for preventing body odor
- Deodorants
Deodorants / Other products for preventing body odor
Required Documentation
- Documents required for manufacturing (import) product approval (notification) of quasi-drugs
-
- Application form
- Business registration certificate
- Notification of quasi-drugs manufacturing or notification of drug and quasi-drugs import business
- Data about the representative, such as the representative's health examination certificate
- Approval of manufacturing (import) manager (※ limited to goods in item 1) or copy of pharmacist's license
- Certified copy of corporate registration: copy (including expungement) or self-inquiry
- Building registry (or factory registration certificate), lease agreement (including factory drawings)
- Facility statement (including manufacturing facility statement and quality control facility statement, floor plan, etc.)
※In the case of quality consignment, a copy of the laboratory, quality consignment contract - One or more manufacturing and sales (import) product declaration or manufacturing and sales (import) product permission application
※You shall apply for a manufacturing notification and a manufacturing sale item license (notification) at the same time - (If applicable) other 5requested documents, etc.
- Documents required for registration of「cosmetics」manufacturing business
-
- Application form
- Business registration certificate
- A doctor's medical certificate
※ An original medical certificate showing that “not a mentally ill person or drug or other toxic substance addictsby Article 3 (1) of the Mental Health Act” - Specifications of facilities (building management ledger, statement of the manufacturing facility and test facility, etc.)
- (If applicable) other requested documents, etc.
- Documents required for registration of 「cosmetics」manufacturing and sales business
-
- Application form
- Business registration certificate
- A doctor's medical certificate
※ An original medical certificate showing that “not a mentally ill person or drug or other toxic substance addictsby Article 3 (1) of the Mental Health Act” - Documents related to qualification standards for manufacturing and sales managers (graduation certificate, transcript, career certificate, etc.)
- Safety management standards and quality management standards
- In-house quality management facility statement or quality management consignment contract
- (If applicable) other requested documents, etc.
Process
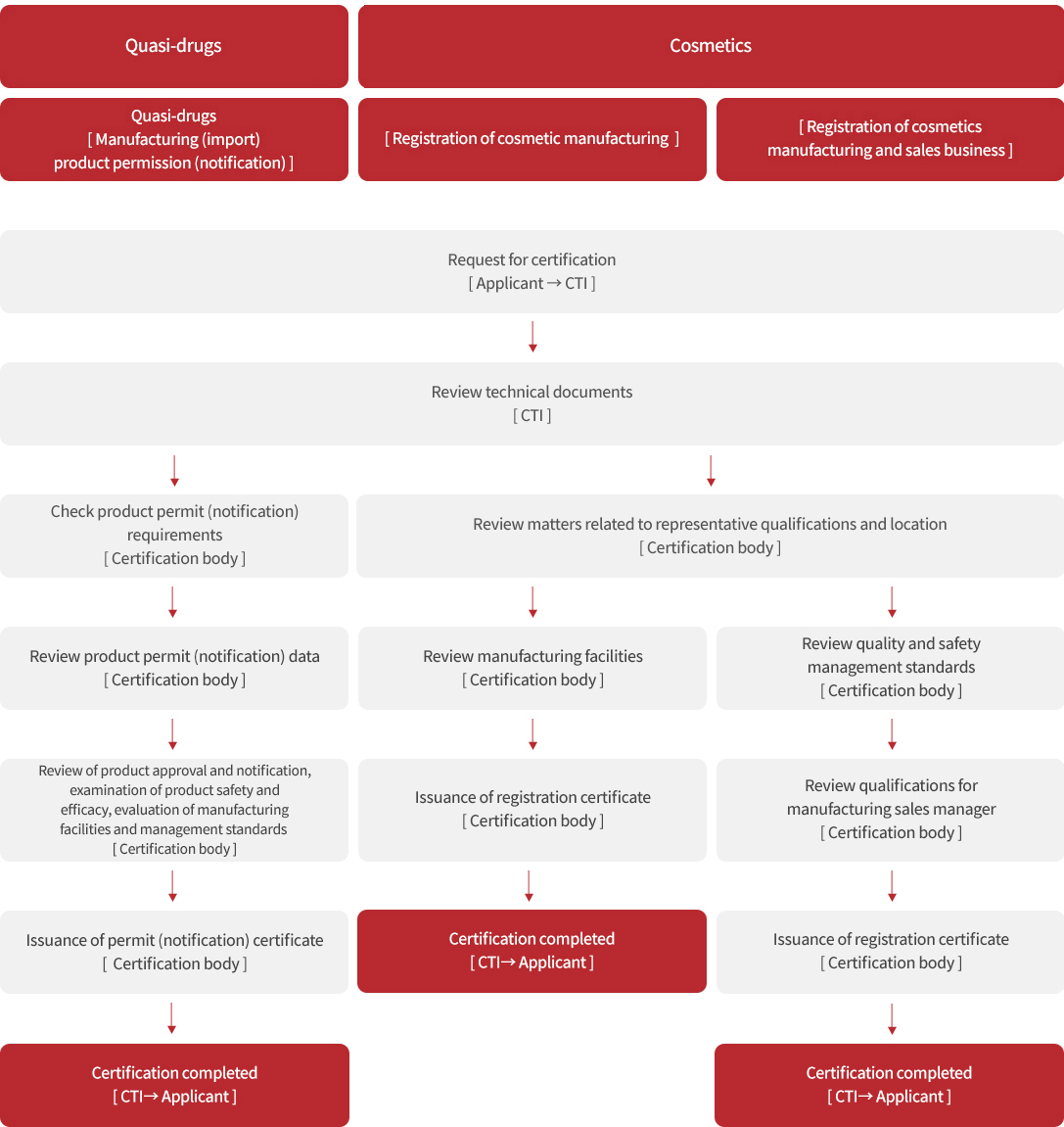
※ In case of nonconformity, the nonconformity (document or sample) needs to be supplemented.
Remarks
Quasi-drugs
- Validity period
- No validity period
- Penalty
- Article 93 (Penalty) of the「Pharmaceutical Affairs Act」A person who has obtained permission or failed to notify, or a person who has obtained permission for modification or failure to notify modification in violation of paragraphs 1 through 4 or 9 of Article 31 shall be punished by imprisonment for not more than five years or a fine not exceeding 50 million won.
Cosmetics
- Validity period
- 3 years from the date of certification
- Penalty
- ① Article 36 (Penalty) of the「Cosmetics Act」 A person who has obtained certification through false or unlawful methods under paragraph 3, item 1 of Article 14-2 shall be punished by imprisonment for not more than three years or by a fine not exceeding 30 million won.
- ② Article 36 (Penalty) of the「Cosmetics Act」A person who failed to register cosmetics manufacturing business or responsible cosmetics sales business under paragraph 1 of Article 3 shall be punished by imprisonment for not more than three years or by a fine not exceeding 30 million won.

